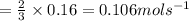Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1


Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2
You know the right answer?
Consider the reaction, 2 d(g) + 3 e(g) + f(g) => 2 g(g) + h(g) when e is decreasing at 0.16 mol/...
Questions





Mathematics, 22.12.2019 00:31

Mathematics, 22.12.2019 00:31

Mathematics, 22.12.2019 00:31



Health, 22.12.2019 00:31



Mathematics, 22.12.2019 00:31

Biology, 22.12.2019 00:31

English, 22.12.2019 00:31

Mathematics, 22.12.2019 00:31

Mathematics, 22.12.2019 00:31

Chemistry, 22.12.2019 00:31


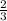 moles of g formed
moles of g formed