
Chemistry, 11.07.2019 19:00 hayesvolcano
A1.42-g sample of a pure compound with formula m2so4 was dissolved in water and treated with an ex- cess of aqueous calcium chloride, resulting in the precipi- tation of all the sulfate ions as calcium sulfate. the pre- cipitate was collected, dried, and found to weigh 1.36 g. determine the atomic mass of m and identify m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

You know the right answer?
A1.42-g sample of a pure compound with formula m2so4 was dissolved in water and treated with an ex-...
Questions

Mathematics, 04.11.2021 07:00

Mathematics, 04.11.2021 07:00



English, 04.11.2021 07:00


History, 04.11.2021 07:00

English, 04.11.2021 07:00




Mathematics, 04.11.2021 07:00



Mathematics, 04.11.2021 07:00


Mathematics, 04.11.2021 07:00


English, 04.11.2021 07:00

Mathematics, 04.11.2021 07:00

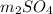 with calcium chloride can be shown as-
with calcium chloride can be shown as-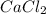 →
→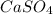 ↓+2mCl. The molecular weight of
↓+2mCl. The molecular weight of 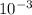 moles of calcium sulfate.
moles of calcium sulfate.


