
Chemistry, 11.07.2019 19:00 cjdolce9790
The element lead (pb) consists of four naturally occurring isotopes with atomic masses 203.97302,205.97444,206.97587, n 207.97663 amu. the relative abundances of these four isotopes are 1.4,24.1,22.1, and 52.4 %, respectively. write the most common isotope of lead in two ways

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 00:30
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1

Chemistry, 23.06.2019 04:20
Calculate the mass of 0.750 mol of the following substance. na3po4.
Answers: 1
You know the right answer?
The element lead (pb) consists of four naturally occurring isotopes with atomic masses 203.97302,205...
Questions











Chemistry, 01.09.2020 21:01







Biology, 01.09.2020 21:01

Chemistry, 01.09.2020 21:01

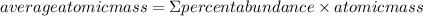
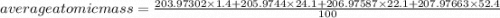
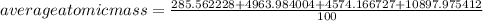
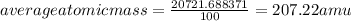
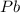 . The atomic number of
. The atomic number of 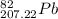 and Lead - 207.22 amu.
and Lead - 207.22 amu.

