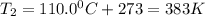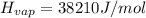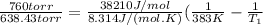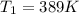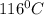Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
Find the boiling point temperature at 760 torr of an isomer of octane, c8h18, if its enthalpy of vap...
Questions

Chemistry, 23.02.2021 02:50


Mathematics, 23.02.2021 02:50

Mathematics, 23.02.2021 02:50


Mathematics, 23.02.2021 02:50


Mathematics, 23.02.2021 02:50

English, 23.02.2021 02:50

Geography, 23.02.2021 02:50




Mathematics, 23.02.2021 02:50

Spanish, 23.02.2021 02:50

Mathematics, 23.02.2021 02:50

Mathematics, 23.02.2021 02:50



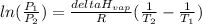
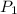 is the vapor pressure at boiling point = 760 torr
is the vapor pressure at boiling point = 760 torr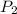 is the vapor pressure at T_{2} =638.43 torr
is the vapor pressure at T_{2} =638.43 torr