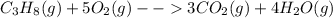During a chemical reaction, 25.0 g of propane burns with 90.7 g of oxygen to form carbon dioxide and water. what mass of carbon dioxide and water is formed? during a chemical reaction, 25.0 g of propane burns with 90.7 g of oxygen to form carbon dioxide and water. what mass of carbon dioxide and water is formed? 115.7 g 90.7 g 65.7 g 25.0 g

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 05:30
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1

Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
During a chemical reaction, 25.0 g of propane burns with 90.7 g of oxygen to form carbon dioxide and...
Questions




Mathematics, 30.06.2021 19:40



Health, 30.06.2021 19:40

Social Studies, 30.06.2021 19:40





Mathematics, 30.06.2021 19:40


Mathematics, 30.06.2021 19:40

Computers and Technology, 30.06.2021 19:40

Mathematics, 30.06.2021 19:40

Mathematics, 30.06.2021 19:40