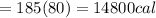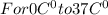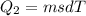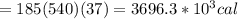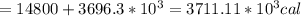Asports trainer applies an ice bag to the back of an injured athlete. calculate the heat in kcal that is absorbed if 185 g of ice at 0.0 ∘c is placed in an ice bag, melts, and rises to body temperature of 37.0 ∘ c. (for water, 80. cal (334 j) is needed to melt 1 g of ice or must be removed to freeze 1 g of water.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 01:00
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2
You know the right answer?
Asports trainer applies an ice bag to the back of an injured athlete. calculate the heat in kcal tha...
Questions



Mathematics, 05.11.2019 16:31



Social Studies, 05.11.2019 16:31



Mathematics, 05.11.2019 16:31

Social Studies, 05.11.2019 16:31

History, 05.11.2019 16:31



English, 05.11.2019 16:31



Mathematics, 05.11.2019 16:31

Mathematics, 05.11.2019 16:31

English, 05.11.2019 16:31

Mathematics, 05.11.2019 16:31