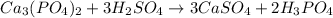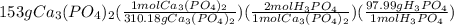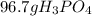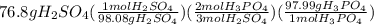Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 22:30
Why is it possible for different microorganisms to extract energy not only from carbohydrates and other biological molecules but from a large variety of substances?
Answers: 1
You know the right answer?
Suppose the reaction ca3(po4)2 + 3h2so4 ® 3caso4 + 2h3po4 is carried out starting with 153 g of ca3(...
Questions



Mathematics, 28.06.2019 18:30




Biology, 28.06.2019 18:30


Chemistry, 28.06.2019 18:30

Mathematics, 28.06.2019 18:30


Mathematics, 28.06.2019 18:30

Mathematics, 28.06.2019 18:30


History, 28.06.2019 18:30


History, 28.06.2019 18:30

Mathematics, 28.06.2019 18:30

Mathematics, 28.06.2019 18:30

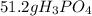 .
.