
Choose the correct interpretation of this result in terms of the law of definite proportion. the composition of a substance depends on the total number of atoms and not on the composition of the mixture from which it was formed. the composition of a substance depends on the numbers of atoms of each element making up the compound (depends on the formula of the compound) and not on the composition of the mixture from which it was formed. the composition of a substance depends on the composition of the mixture from which it was formed and not on the numbers of atoms of each element making up the compound (not on the formula of the compound). the composition of a mixture depends on the numbers of atoms of each element making up the mixture and not on the composition of the substance from which it was formed.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2
You know the right answer?
Choose the correct interpretation of this result in terms of the law of definite proportion. the com...
Questions

Arts, 31.05.2021 04:10

Mathematics, 31.05.2021 04:10


Physics, 31.05.2021 04:10

Social Studies, 31.05.2021 04:10

Social Studies, 31.05.2021 04:10

Mathematics, 31.05.2021 04:10


Mathematics, 31.05.2021 04:10

Arts, 31.05.2021 04:10



English, 31.05.2021 04:10





Mathematics, 31.05.2021 04:10

Mathematics, 31.05.2021 04:10

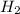 (g) +
(g) + 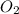 (g) = 2
(g) = 2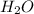 (l). Here the origin of the components i.e. hydrogen and oxygen does not matter it depends upon the number of atoms present in the reactant and product. Thus among all the other interpretations- The composition of a substance depends on the total number of atoms and not on the composition of the mixture from which it was formed. This is correct.
(l). Here the origin of the components i.e. hydrogen and oxygen does not matter it depends upon the number of atoms present in the reactant and product. Thus among all the other interpretations- The composition of a substance depends on the total number of atoms and not on the composition of the mixture from which it was formed. This is correct. 

