
Bromine reacts with nitric oxide to form nitrosyl bromide as shown in this reaction: br2(g) + 2 no(g) → 2 nobr(g) a possible mechanism for this overall reaction is shown below. no(g) + br2(g) br2(g) (fast step; keq = k1/k−1) k2 nobr(g) + no(g) → 2 nobr(g) (slow step) what is the rate law for formation of nobr in terms of reactants based on this mechanism

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
Bromine reacts with nitric oxide to form nitrosyl bromide as shown in this reaction: br2(g) + 2 no(...
Questions



Spanish, 28.01.2021 23:50

Mathematics, 28.01.2021 23:50

Mathematics, 28.01.2021 23:50



French, 28.01.2021 23:50


Chemistry, 28.01.2021 23:50

Mathematics, 28.01.2021 23:50



Mathematics, 28.01.2021 23:50

Biology, 28.01.2021 23:50


Chemistry, 28.01.2021 23:50

Social Studies, 28.01.2021 23:50

Mathematics, 28.01.2021 23:50

Mathematics, 28.01.2021 23:50

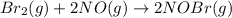
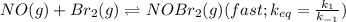
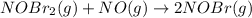 (slow step
(slow step 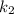 )
)![r_{1}=k_{1}[NO][Br_{2}]-k_{-1}[NOBr_{2}]](/tpl/images/0077/5023/9bc26.png)
![r_{2}=k_{2}[NOBr_{2}] [NO]](/tpl/images/0077/5023/6e659.png)
![[NOBr_{2}]](/tpl/images/0077/5023/48931.png) takes place in this reaction.
takes place in this reaction.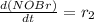
![k_{2}[NOBr_{2}][NO]](/tpl/images/0077/5023/433e8.png) (1)
(1)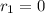
![k_{1}[NO][Br_{2}]= k_{-1}[NOBr_{2}]](/tpl/images/0077/5023/8345a.png)
![[NOBr_{2}] = \frac{k_{1}}{k_{-1}}[NO][Br_{2}]](/tpl/images/0077/5023/ee42f.png)
![\frac{d(NOBr)}{dt}=k_{2}[NOBr_{2}][NO]](/tpl/images/0077/5023/ff99b.png)
![k_{2} \frac{k_{1}}{k_{-1}}[NO][Br_{2}][NO]](/tpl/images/0077/5023/f5111.png)
![\frac{k_{1}k_{2}}{k_{-1}}[NO]^{2}[Br_{2}]](/tpl/images/0077/5023/427a8.png)
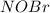 in terms of reactants is given by
in terms of reactants is given by 

