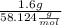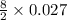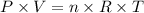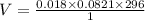Butane, c4h10, is a component of natural gas that is used as fuel for cigarette lighters. the balanced equation of the complete combustion of butane is 2c4h10(g)+13o2(g)→8co2(g)+10h2o(l) at 1.00 atm and 23 ∘c, what is the volume of carbon dioxide formed by the combustion of 1.60 g of butane?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1
You know the right answer?
Butane, c4h10, is a component of natural gas that is used as fuel for cigarette lighters. the balanc...
Questions


Mathematics, 25.11.2020 08:40

Social Studies, 25.11.2020 08:40

Mathematics, 25.11.2020 08:40



History, 25.11.2020 08:40

Health, 25.11.2020 08:40


Health, 25.11.2020 08:40

Chemistry, 25.11.2020 08:40

Mathematics, 25.11.2020 08:40


Mathematics, 25.11.2020 08:40



History, 25.11.2020 08:40

Business, 25.11.2020 08:40

Mathematics, 25.11.2020 08:40