
Chemistry, 11.07.2019 15:00 wittlemarie
At a certain temperature, the solubility of n2 gas in water at 3.08 atm is 72.5 mg of n2 gas/100 g water . calculate the solubility of n2 gas in water, at the same temperature, if the partial pressure of n2 gas over the solution is increased from 3.08 atm to 8.00 atm . express your answer numerically to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Llama have 74 chromosomes how many chromosomes will they be found in their gametes explain how you know
Answers: 2

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
At a certain temperature, the solubility of n2 gas in water at 3.08 atm is 72.5 mg of n2 gas/100 g w...
Questions



Mathematics, 21.07.2019 00:10


Mathematics, 21.07.2019 00:10



Mathematics, 21.07.2019 00:10

Spanish, 21.07.2019 00:10



Mathematics, 21.07.2019 00:10


Spanish, 21.07.2019 00:10

Mathematics, 21.07.2019 00:10



Mathematics, 21.07.2019 00:10




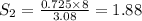
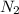 gas in 1 g of water or, 188 mg of tex]N_{2}[/tex] gas in 100 g of water.
gas in 1 g of water or, 188 mg of tex]N_{2}[/tex] gas in 100 g of water.

