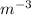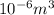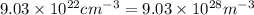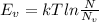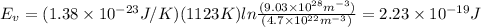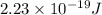Chemistry, 11.07.2019 11:30 litzyguzman13
Calculate the energy for vacancy formation in nickel (ni), given that the equilibrium number of vacancies at 850°c (1123 k) is 4.7 × 1022 m–3. the atomic weight and density (at 850°c) for ni are, respectively, 58.69 g/mol and 8.80 g/cm3.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 24.06.2019 03:30
Based on the data given and a rate constant of 0.031 m−1⋅min−1, calculate the time at which the concentration of reactant a will be 0.175
Answers: 1
You know the right answer?
Calculate the energy for vacancy formation in nickel (ni), given that the equilibrium number of vaca...
Questions

Mathematics, 09.12.2020 19:10


Engineering, 09.12.2020 19:10

Mathematics, 09.12.2020 19:10







Social Studies, 09.12.2020 19:10

History, 09.12.2020 19:10

Spanish, 09.12.2020 19:10




Advanced Placement (AP), 09.12.2020 19:10



Biology, 09.12.2020 19:10

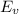 can be calculated as:
can be calculated as: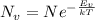
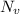 is equilibrium number of vacancies, N is number of atomic sites per unit vacancies, k is Boltzmann constant, T is temperature.
is equilibrium number of vacancies, N is number of atomic sites per unit vacancies, k is Boltzmann constant, T is temperature.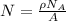
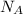 is Avogadro's number and A is atomic weight.
is Avogadro's number and A is atomic weight.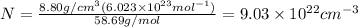
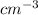 to
to