
Chemistry, 11.07.2019 07:30 j1theking18
Acompound contains only carbon, hydrogen, and oxygen. combustion of 11.75 mg of the compound yields 17.61 mg co2 and 4.81 mg h2o. the molar mass of the compound is 176.1 g/mol. what are the empirical and molecular formulas of the compound? (type your answer using the format co2 for co2.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


You know the right answer?
Acompound contains only carbon, hydrogen, and oxygen. combustion of 11.75 mg of the compound yields...
Questions


Mathematics, 19.05.2020 02:06

English, 19.05.2020 02:06



History, 19.05.2020 02:06


History, 19.05.2020 02:06

Mathematics, 19.05.2020 02:06


Biology, 19.05.2020 02:06

Mathematics, 19.05.2020 02:06

Biology, 19.05.2020 02:06

Mathematics, 19.05.2020 02:06



Mathematics, 19.05.2020 02:06


Mathematics, 19.05.2020 02:06

Mathematics, 19.05.2020 02:06

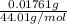
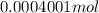
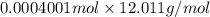
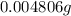
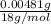

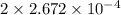
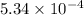
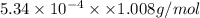
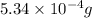
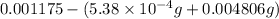
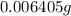
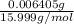
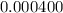
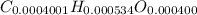
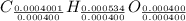
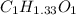
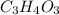 (empirical fomula)
(empirical fomula)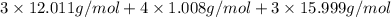
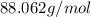
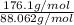
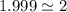
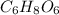
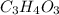 and molecular formula is
and molecular formula is 

