
Chemistry, 11.07.2019 07:30 emilybutler
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decomposed the sulfur dioxide produced 3.49 g oxygen and 3.50 g sulfur, while the sulfur trioxide produced 6.75 g oxygen and 4.50 g sulfur. calculate the mass of oxygen per gram of sulfur for each sample and show that these results are consistent with the law of multiple proportions.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1
You know the right answer?
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decompose...
Questions




Social Studies, 12.09.2019 02:10

History, 12.09.2019 02:10






History, 12.09.2019 02:10




Mathematics, 12.09.2019 02:10


Engineering, 12.09.2019 02:10

Computers and Technology, 12.09.2019 02:10


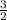 O₂
O₂

