
This reaction shows the complete combustion of octane, c8h18, a component of gasoline. 2 c8h18(l) + 25 o2(g) 16 co2(g) + 18 h2o(l) (a) how many moles of o2 are needed to burn 1.60 mol of c8h18? mol (b) how many moles of co2 are produced when 0.19 mol of c8h18 are burned? mol (c) how many grams of o2 are needed to burn 1.15 g of c8h18? g

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1
You know the right answer?
This reaction shows the complete combustion of octane, c8h18, a component of gasoline. 2 c8h18(l) +...
Questions





Mathematics, 28.08.2019 23:50



Mathematics, 28.08.2019 23:50


Mathematics, 28.08.2019 23:50


Mathematics, 28.08.2019 23:50

History, 28.08.2019 23:50

Mathematics, 28.08.2019 23:50


Mathematics, 29.08.2019 00:00

Mathematics, 29.08.2019 00:00



Social Studies, 29.08.2019 00:00

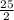 O₂→ 8 CO₂ + 9 H₂O.
O₂→ 8 CO₂ + 9 H₂O. 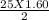 mole= 20 moles.
mole= 20 moles.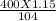 g= 4.42 g.
g= 4.42 g.

