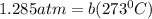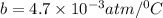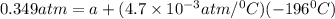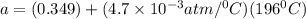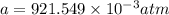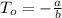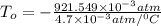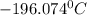Chemistry, 11.07.2019 07:00 lauren21bunch
In a student experiment, a constant-volume gas thermometer is calibrated in liquid nitrogen (−196°c ) and in boiling ethyl alcohol (77°c). the separate pressures are 0.349 atm and 1.634 atm. hint: use the linear relationship p = a + bt, where a and b are constants. (a) what value of absolute zero does the calibration yield?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
In a student experiment, a constant-volume gas thermometer is calibrated in liquid nitrogen (−196°c...
Questions



Spanish, 30.10.2019 19:31


Mathematics, 30.10.2019 19:31




Mathematics, 30.10.2019 19:31


Mathematics, 30.10.2019 19:31









English, 30.10.2019 19:31

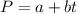
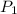 (Pressure of liquid nitrogen) =
(Pressure of liquid nitrogen) =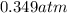
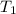 (Temperature of liquid nitrogen) =
(Temperature of liquid nitrogen) =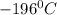
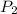 (Pressure of ethyl alcohol) =
(Pressure of ethyl alcohol) =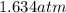
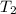 (Temperature of ethyl alcohol) =
(Temperature of ethyl alcohol) =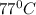
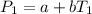
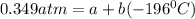 (1)
(1)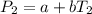
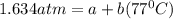 (2)
(2)