
Chemistry, 11.07.2019 07:00 kaylamaisonettt
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 4.15 ∘c. what is the molal concentration of glucose in this solution? assume that the freezing point of pure water is 0.00 ∘c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1


Chemistry, 23.06.2019 06:30
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1
You know the right answer?
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 4.15 ∘c. what is the molal concentration...
Questions

Mathematics, 04.12.2020 16:30



Mathematics, 04.12.2020 16:30




Biology, 04.12.2020 16:30






Advanced Placement (AP), 04.12.2020 16:30



English, 04.12.2020 16:30



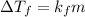 -(1)
-(1)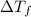 is depression of freezing point,
is depression of freezing point, 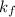 is freezing point depression constant and
is freezing point depression constant and 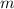 is molality.
is molality.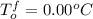 (given)
(given)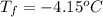 (given)
(given)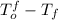
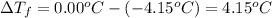
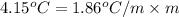
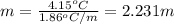
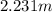 .
.

