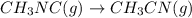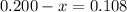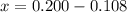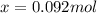Chemistry, 11.07.2019 07:00 aurelio1121
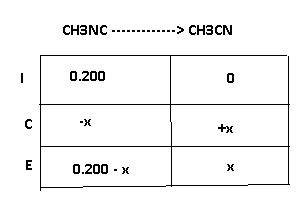
At elevated temperatures, methylisonitrile (ch3nc) isomerizes to acetonitrile (ch3cn): ch3nc (g) \rightarrow → ch3cn(g) at the start of an experiment, there are 0.200 mol of reactant and 0 mol of product in the reaction vessel. after 25 min, 0.108 mol of reactant (ch3nc) remain. there are mol of product (ch3cn) in the reaction vessel.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
At elevated temperatures, methylisonitrile (ch3nc) isomerizes to acetonitrile (ch3cn): ch3nc (g) \r...
Questions


Business, 08.09.2020 14:01





Chemistry, 08.09.2020 14:01

English, 08.09.2020 14:01


Computers and Technology, 08.09.2020 14:01




Mathematics, 08.09.2020 14:01



Mathematics, 08.09.2020 14:01

History, 08.09.2020 14:01

Computers and Technology, 08.09.2020 14:01