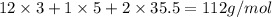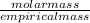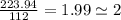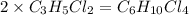Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
An unknown compound with a molar mass of 223.94 g/mol consists of 32.18% c, 4.50% h, and 63.32% cl....
Questions

Mathematics, 11.03.2021 16:50

Social Studies, 11.03.2021 16:50

Mathematics, 11.03.2021 16:50

Mathematics, 11.03.2021 16:50

Mathematics, 11.03.2021 16:50



Mathematics, 11.03.2021 16:50



Mathematics, 11.03.2021 16:50




Mathematics, 11.03.2021 16:50

Mathematics, 11.03.2021 16:50

Mathematics, 11.03.2021 16:50



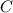 :
: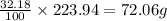
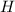 :
:
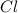 :
: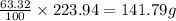
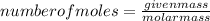
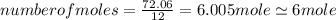
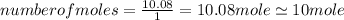
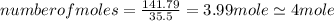
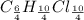

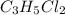 .
.