Chemistry, 10.07.2019 21:30 SKYBLUE1015
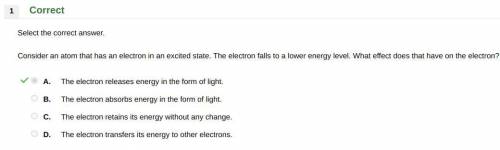
Consider an atom that has an electron in an excited state. the electron falls to a lower energy level. what effect does that have on the electron? a. the electron releases energy in the form of light. b. the electron absorbs energy in the form of light. c. the electron retains its energy without any change. d. the electron transfers its energy to other electrons.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
Consider an atom that has an electron in an excited state. the electron falls to a lower energy leve...
Questions






Mathematics, 23.08.2019 06:30



Chemistry, 23.08.2019 06:30

Mathematics, 23.08.2019 06:30



Mathematics, 23.08.2019 06:30

Social Studies, 23.08.2019 06:30

History, 23.08.2019 06:30




Chemistry, 23.08.2019 06:30

Mathematics, 23.08.2019 06:30