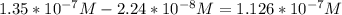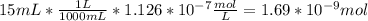Chemistry, 10.07.2019 21:00 deanlmartin
In a typical analysis, 15 ml of an aqueous solution containing an unknown amount of acetylcholine had a ph of 7.65. when incubated with acetylcholinesterase, the ph of the solution decreased to 6.87. assuming there was no buffer in the assay mixture, determine the number of moles of acetylcholine in the 15 ml sample.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1


Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
In a typical analysis, 15 ml of an aqueous solution containing an unknown amount of acetylcholine ha...
Questions


English, 11.09.2021 03:10


Mathematics, 11.09.2021 03:10


English, 11.09.2021 03:10

German, 11.09.2021 03:10

Mathematics, 11.09.2021 03:10

Mathematics, 11.09.2021 03:10





Mathematics, 11.09.2021 03:10

Mathematics, 11.09.2021 03:10


Biology, 11.09.2021 03:10



Mathematics, 11.09.2021 03:10

![[H_{3}O^{+}]=10^{-7.65}=2.24*10^{-8} M](/tpl/images/0074/6124/2f1c4.png)
![[H_{3}O^{+}]=10^{-6.87}=1.35*10^{-7} M](/tpl/images/0074/6124/010e5.png)