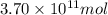Chemistry, 10.07.2019 21:00 hapjajsjjz3738
The annual production of sulfur dioxide from burning coal and fossil fuels, auto exhaust, and other sources is about 26 million tons. the equation for the reaction is s(s) + o2(g) → so2(g) how much sulfur (in tons), present in the original materials, would result in that quantity of so2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1


Chemistry, 23.06.2019 16:30
Two like-charged particles are placed close to each other. how would the force of repulsion be affected if the charge on one of the particles is doubled and that on the other is reduced to half the original value?
Answers: 1

You know the right answer?
The annual production of sulfur dioxide from burning coal and fossil fuels, auto exhaust, and other...
Questions

Mathematics, 31.08.2019 11:50

Social Studies, 31.08.2019 11:50


Mathematics, 31.08.2019 11:50

Mathematics, 31.08.2019 11:50

Mathematics, 31.08.2019 11:50


Social Studies, 31.08.2019 11:50


Mathematics, 31.08.2019 11:50

Spanish, 31.08.2019 11:50

Mathematics, 31.08.2019 11:50

Mathematics, 31.08.2019 11:50

Biology, 31.08.2019 11:50


Mathematics, 31.08.2019 11:50


Advanced Placement (AP), 31.08.2019 11:50


Computers and Technology, 31.08.2019 11:50

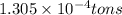
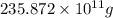 (Conversion factor:
(Conversion factor: 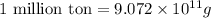 )
)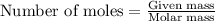 .....(1)
.....(1)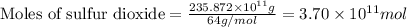
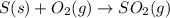
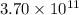 moles of sulfur dioxide will be produced by =
moles of sulfur dioxide will be produced by = 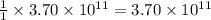 moles of sulfur.
moles of sulfur.