Chemistry, 10.07.2019 20:30 rleiphart1
A59.4-mg sample of the compound x4o6 contains 14.4 mg of oxygen atoms. what is the atomic mass of element x?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1
You know the right answer?
A59.4-mg sample of the compound x4o6 contains 14.4 mg of oxygen atoms. what is the atomic mass of el...
Questions

Mathematics, 18.11.2020 23:40



Mathematics, 18.11.2020 23:40

Health, 18.11.2020 23:40


Computers and Technology, 18.11.2020 23:40


English, 18.11.2020 23:40

Chemistry, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

History, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

History, 18.11.2020 23:40

Mathematics, 18.11.2020 23:40

Advanced Placement (AP), 18.11.2020 23:40

Mathematics, 18.11.2020 23:40


Physics, 18.11.2020 23:40

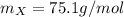
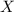 's percent as shown below:
's percent as shown below: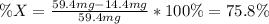
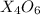 as follows:
as follows: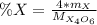
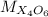 is the molar mass of the given compound and
is the molar mass of the given compound and 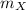 the atomic mass of
the atomic mass of