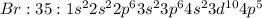Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1


Chemistry, 23.06.2019 14:00
During an acid-base titration, when do the contents of the beaker consist of only water, a salt, and a trace of indicator?
Answers: 2

Chemistry, 23.06.2019 21:50
Absalon adds 1 g of salt to 1 l of room temperature water (25 °c). then, he starts a timer and observes what happens. he notices that it takes 1 minute for the salt to dissolve. he decides to repeat his experiment, and he adds 1 g of salt to another 1 l of room temperature water (25 °c). after he adds the salt, he starts a timer. but, instead of watching the salt dissolve, he stirs the salt and water with a spoon until it dissolves. he notices that it only takes 30 seconds for the salt to dissolve in his second experiment. why does the salt dissolve faster in absalon's second experiment? stirring the salt and water increases the polarity of the water molecules, which causes the ionic bonds of the salt to break. stirring the salt and water increases particle motion, which causes more collisions to occur between the water and salt. stirring the salt and water increases the surface area of the water, which causes more collisions to occur between the water and salt. stirring the salt and water increases the pressure on the solution, which causes the ionic bonds of the salt to break.
Answers: 1
You know the right answer?
Write the ground state electron configuration of br using the noble-gas shorthand notation....
Questions

Mathematics, 05.01.2020 21:31


Mathematics, 05.01.2020 21:31

Health, 05.01.2020 21:31


Mathematics, 05.01.2020 21:31



English, 05.01.2020 21:31

Mathematics, 05.01.2020 21:31


History, 05.01.2020 21:31





Biology, 05.01.2020 21:31



Physics, 05.01.2020 21:31

![Br:35:[Ar]4s^23d^{10}4p^5](/tpl/images/0073/9953/a0b36.png)