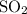Chemistry, 10.07.2019 17:00 kylieweeks052704
Part a choose the pair of substances that are most likely to form a homogeneous solution. choose the pair of substances that are most likely to form a homogeneous solution. co and c6h14 nh2ch3 and ch4 cbr4 and sf2 nf3 and so2 none of the pairs above will form a homogeneous solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2
You know the right answer?
Part a choose the pair of substances that are most likely to form a homogeneous solution. choose the...
Questions


History, 08.10.2021 18:50


Mathematics, 08.10.2021 18:50

Mathematics, 08.10.2021 18:50


English, 08.10.2021 18:50


English, 08.10.2021 18:50




History, 08.10.2021 18:50


Mathematics, 08.10.2021 18:50


Mathematics, 08.10.2021 18:50

Mathematics, 08.10.2021 18:50

Computers and Technology, 08.10.2021 18:50

Mathematics, 08.10.2021 18:50

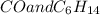 : CO is a polar gas and
: CO is a polar gas and 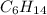 is a non polar liquid. Therefore, this solution will not be homogeneous.
is a non polar liquid. Therefore, this solution will not be homogeneous.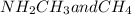 :
: 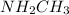 is a polar liquid while methane is a non polar gas. So, this solution will not be homogeneous.
is a polar liquid while methane is a non polar gas. So, this solution will not be homogeneous.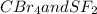 :
:  is a non polar solid while
is a non polar solid while 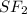 is a polar gas. So, they cannot form homogeneous solution.
is a polar gas. So, they cannot form homogeneous solution.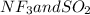 :
: 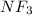 and
and 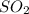 are both gases. So, they form a uniform homogeneous solution. Both can uniformly interact as they both are polar compounds.
are both gases. So, they form a uniform homogeneous solution. Both can uniformly interact as they both are polar compounds.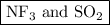 is the pair that is most likely to form a homogeneous solution.
is the pair that is most likely to form a homogeneous solution.
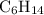
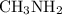 and
and 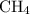
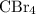 and
and 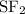
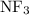 and
and