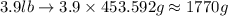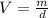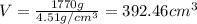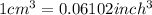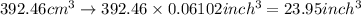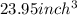Chemistry, 10.07.2019 17:00 Emptypockets451
The density of titanium is 4.51 g/cm3. what is the volume (in cubic inches) of 3.9 lb of titanium?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:30
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
The density of titanium is 4.51 g/cm3. what is the volume (in cubic inches) of 3.9 lb of titanium?...
Questions

Mathematics, 03.09.2021 07:00

English, 03.09.2021 07:00




Biology, 03.09.2021 07:00

History, 03.09.2021 07:00


Biology, 03.09.2021 07:00


English, 03.09.2021 07:00

Advanced Placement (AP), 03.09.2021 07:00

History, 03.09.2021 07:00

English, 03.09.2021 07:00

Mathematics, 03.09.2021 07:00



History, 03.09.2021 07:00


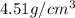 and mass given is 3.9 lb. First convert mass from pound to gram as follows:
and mass given is 3.9 lb. First convert mass from pound to gram as follows: