
10.00 ml of the final acid solution is reacted with excess barium chloride to produce a precipitate of barium sulfate (fw: 233.4 g/mol). the dry solid weighs 0.397 g. use this mass and the dilution volumes to calculate the actual molarity of the sulfuric acid in the initial solution. (previous question ask: 10.00 ml of approximately 6 m sulfuric acid is transferred to a 100ml volumetric flask and diluted to the mark with distilled water and mixed. then 10.00 ml of this solution was further diluted to 100 ml. the molarity of the final solution was 0.06 m).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1


Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1
You know the right answer?
10.00 ml of the final acid solution is reacted with excess barium chloride to produce a precipitate...
Questions


Mathematics, 10.12.2020 23:40

Computers and Technology, 10.12.2020 23:40




Chemistry, 10.12.2020 23:40

Mathematics, 10.12.2020 23:40

Mathematics, 10.12.2020 23:40

Mathematics, 10.12.2020 23:40

English, 10.12.2020 23:40


Mathematics, 10.12.2020 23:40




Mathematics, 10.12.2020 23:40


Mathematics, 10.12.2020 23:40

Mathematics, 10.12.2020 23:40

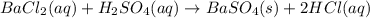
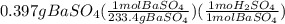
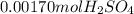
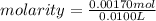
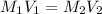
![Y=\frac{0.170M*100mL}{10.0mL}Y = 1.70MLet's do the similar calculations to find out the actual molarity of the original acid solution. Let's say the molarity of the original acid solution is X. 10.0 mL of it were taken and diluted to 100 mL on adding water. The molarity is 1.70M as is calculated in the above step. Let's plug in the values in the molarity equation again to solve it for X as:X(10.0mL) = 1.70M(100mL)[tex]X=\frac{1.70M*100mL}{10.0mL}](/tpl/images/0073/9434/d3677.png)


