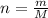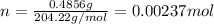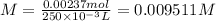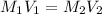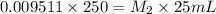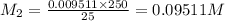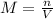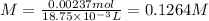Chemistry, 10.07.2019 16:30 bugsbunny27
If a 0.4856 gram sample of khp is dissolved in sufficient water to prepare 250 ml of solution, and 25 ml of the solution requires 18.76ml of sodium hydroxide solution to reach the equivalence point, what is the molarity of the sodium hydroxide?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
If a 0.4856 gram sample of khp is dissolved in sufficient water to prepare 250 ml of solution, and 2...
Questions

Mathematics, 30.06.2019 09:20


Health, 30.06.2019 09:20




Mathematics, 30.06.2019 09:20

Advanced Placement (AP), 30.06.2019 09:20

Mathematics, 30.06.2019 09:20


Mathematics, 30.06.2019 09:20


Mathematics, 30.06.2019 09:20





Geography, 30.06.2019 09:20

Mathematics, 30.06.2019 09:20