Chemistry, 10.07.2019 16:30 jmurguia888
Acertain element x has four isotopes. 4.350% of x has a mass of 49.94605 amu. 83.79% of x has a mass of 51.94051 amu. 9.500% of x has a mass of 52.94065 amu. 2.360% of x has a mass of 53.93888 amu. what is the average atomic mass of element x? express your answer numerically to four significant figures. view available hint(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 11:00
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2
You know the right answer?
Acertain element x has four isotopes. 4.350% of x has a mass of 49.94605 amu. 83.79% of x has a mass...
Questions

Mathematics, 13.05.2021 21:10




Mathematics, 13.05.2021 21:10

Chemistry, 13.05.2021 21:10

Physics, 13.05.2021 21:10

Mathematics, 13.05.2021 21:10


Biology, 13.05.2021 21:10






Mathematics, 13.05.2021 21:10

Mathematics, 13.05.2021 21:10

Social Studies, 13.05.2021 21:10


Mathematics, 13.05.2021 21:10

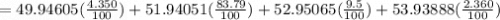
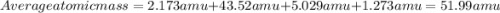
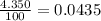
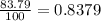
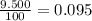
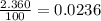
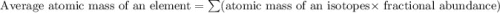
![A=\sum[(49.94605\times 0.0435)+(51.94051\times 0.8379)+(52.94065\times 0.095)+(53.93888\times 0.0236)]](/tpl/images/0073/8885/6e58e.png)