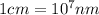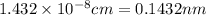Chemistry, 10.07.2019 16:30 tylrmannon
Calculate the radius of a tantalum (ta) atom, given that ta has a bcc crystal structure, a density of 16.6 g/cm3 , and an atomic weight if 180.9 g/mol. (avogadro number, 6.023 ×1023 atoms/mol) (4pts)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
Calculate the radius of a tantalum (ta) atom, given that ta has a bcc crystal structure, a density o...
Questions

Computers and Technology, 06.08.2021 14:00


Mathematics, 06.08.2021 14:00

Mathematics, 06.08.2021 14:00

History, 06.08.2021 14:00

Geography, 06.08.2021 14:00


History, 06.08.2021 14:00

English, 06.08.2021 14:00




Mathematics, 06.08.2021 14:00

Social Studies, 06.08.2021 14:00

English, 06.08.2021 14:00

Mathematics, 06.08.2021 14:00

Social Studies, 06.08.2021 14:00

English, 06.08.2021 14:00

English, 06.08.2021 14:00

Mathematics, 06.08.2021 14:00

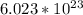
![V_c =[\frac{4R}{\sqrt{3} }]^3](/tpl/images/0073/8832/b260c.png)
![R^3 =\frac{4.87*10^{-23}}{16.6} \\\\R=\sqrt[3]{2.938*10^{-24}} \\\\=1.432*10^{-8](/tpl/images/0073/8832/7fb31.png)
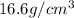 , atomic mass is 180.9 g/mol and Avogadro's number is
, atomic mass is 180.9 g/mol and Avogadro's number is 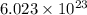 atoms per mol.
atoms per mol.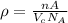
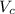 is volume and
is volume and 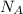 is Avogadro's number .
is Avogadro's number .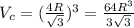
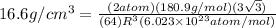
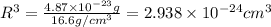
![R=\sqrt[3]{2.938\times 10^{-24}}=1.432\times 10^{-8} cm](/tpl/images/0073/8832/eafa7.png)