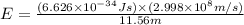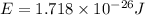Chemistry, 01.10.2019 20:30 kyramks421
Calculate the energy of a photon of wavelength 11.56 meters. (planck’s constant is 6.626 x 10-34 joule seconds; the speed of light is 2.998 x 108 m/s)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
Calculate the energy of a photon of wavelength 11.56 meters. (planck’s constant is 6.626 x 10-34 jou...
Questions

English, 30.08.2020 02:01


Biology, 30.08.2020 02:01

Mathematics, 30.08.2020 02:01

Mathematics, 30.08.2020 02:01

Mathematics, 30.08.2020 02:01


Physics, 30.08.2020 02:01

Mathematics, 30.08.2020 02:01


Social Studies, 30.08.2020 02:01


Geography, 30.08.2020 02:01

History, 30.08.2020 02:01

Business, 30.08.2020 02:01

Mathematics, 30.08.2020 02:01

Mathematics, 30.08.2020 02:01

Spanish, 30.08.2020 02:01


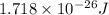
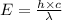
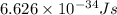
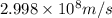
 = wavelength = 11.56 m
= wavelength = 11.56 m