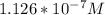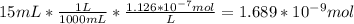Chemistry, 10.07.2019 13:00 jeffcarpenter
In a typical analysis, 15 ml of an aqueous solution containing an unknown amount of acetylcholine had a ph of 7.65. when incubated with acetylcholinesterase, the ph of the solution decreased to 6.87. assuming there was no buffer in the assay mixture, determine the number of moles of acetylcholine in the 15 ml sample.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
In a typical analysis, 15 ml of an aqueous solution containing an unknown amount of acetylcholine ha...
Questions

History, 05.05.2020 14:14


Physics, 05.05.2020 14:14


Spanish, 05.05.2020 14:15

Mathematics, 05.05.2020 14:15


English, 05.05.2020 14:15


Mathematics, 05.05.2020 14:15



Social Studies, 05.05.2020 14:15



Mathematics, 05.05.2020 14:15

History, 05.05.2020 14:15

Mathematics, 05.05.2020 14:15

Mathematics, 05.05.2020 14:15

![[H_{3}O^{+}]=10^{-7.65}=2.24*10^{-8}M](/tpl/images/0073/3031/103c1.png)
![[H_{3}O^{+}]=10^{-6.87}=1.35*10^{-7}M](/tpl/images/0073/3031/b0e50.png)
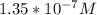 -
-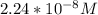 =
=