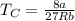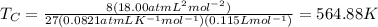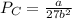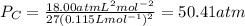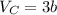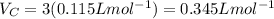Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
The van der waals constants a and b for benzene are 18.00 atm l2 mol−2 and 0.115 l mol−1, respective...
Questions

English, 20.09.2020 01:01

English, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01



Mathematics, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01

History, 20.09.2020 01:01



Physics, 20.09.2020 01:01


English, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Physics, 20.09.2020 01:01