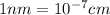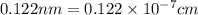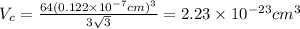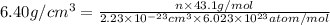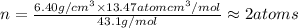Chemistry, 10.07.2019 12:30 savannahvargas512
Ahypothetical alloy has an atomic weight of 43.1 g/mol, a density of 6.40 g/cm3, and an atomic radius of 0.122 nm. determine whether its crystal structure is fcc, bcc, or simple cubic

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1
You know the right answer?
Ahypothetical alloy has an atomic weight of 43.1 g/mol, a density of 6.40 g/cm3, and an atomic radiu...
Questions



Social Studies, 03.08.2019 11:30


Computers and Technology, 03.08.2019 11:30

Computers and Technology, 03.08.2019 11:30




Social Studies, 03.08.2019 11:30


Biology, 03.08.2019 11:30

Computers and Technology, 03.08.2019 11:30

Computers and Technology, 03.08.2019 11:30

Computers and Technology, 03.08.2019 11:30

Computers and Technology, 03.08.2019 11:30

Computers and Technology, 03.08.2019 11:30

Computers and Technology, 03.08.2019 11:30

Computers and Technology, 03.08.2019 11:30

Computers and Technology, 03.08.2019 11:30

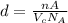 ...... (1)
...... (1)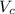 is volume of crystal and
is volume of crystal and 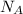 is Avagadro's number.
is Avagadro's number.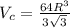
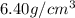 and 0.122 nm respectively.
and 0.122 nm respectively.