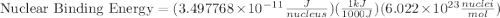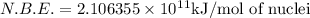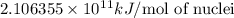Chemistry, 10.07.2019 12:30 janicemaxwell123
How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and neutrons if an aluminum-27 atom has a mass of 26.9815386 amu? (the mass of an electron is 5.485799×10−4 amu, the mass of a proton is 1.0072765 amu, and the mass of a neutron is 1.0086649 amu.) express your answer using six significant figures. g?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1


Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
How much energy must be supplied to break a single aluminum-27 nucleus into separated protons and ne...
Questions



Social Studies, 07.04.2020 17:48


Mathematics, 07.04.2020 17:48


Mathematics, 07.04.2020 17:48

Mathematics, 07.04.2020 17:48






Mathematics, 07.04.2020 17:48

English, 07.04.2020 17:48



History, 07.04.2020 17:48

Chemistry, 07.04.2020 17:48

Chemistry, 07.04.2020 17:48

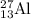 nucleus having 13 protons as it is visible from atomic number and number of neutrons is 14 which is calculated from
nucleus having 13 protons as it is visible from atomic number and number of neutrons is 14 which is calculated from 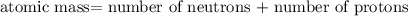 .
. 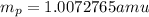 and
and 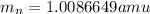 , we can calculate the mass of the isotope.
, we can calculate the mass of the isotope.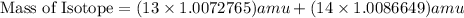
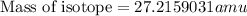
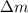 = mass of isotope - atomic mass.
= mass of isotope - atomic mass.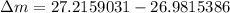

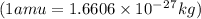
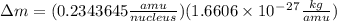
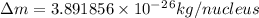
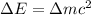
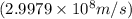
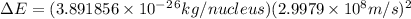
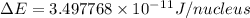
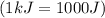 and converting individual particles into moles, we need to multiply it by avagadro's number that is
and converting individual particles into moles, we need to multiply it by avagadro's number that is 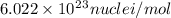 .
.