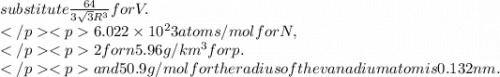Chemistry, 10.07.2019 08:30 dazesreplayy2451
Calculate the radius of a vanadium atom, given that v has a bcc crystal structure, a density of 5.96 g/cm3 , and an atomic weight of 50.9 g/mol

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
Calculate the radius of a vanadium atom, given that v has a bcc crystal structure, a density of 5.96...
Questions


Mathematics, 27.08.2020 01:01





English, 27.08.2020 01:01



Mathematics, 27.08.2020 01:01









Mathematics, 27.08.2020 01:01