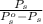An ideal solution is formed from a mixture of the nonvolatile solute urea, co(nh2)2, and methanol, ch3oh. the vapor pressure of pure methanol at 20°c is 89 mmhg. if 6.0 g of urea is mixed with 39.8 g of methanol, calculate the vapor pressure of the methanol solution.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 07:00
Achemist who studies water samples did a demonstration of how to test for lead in water. she added a clear solution of potassium iodide to a clear solution of lead nitrate. then a yellow swirling solid formed in the liquid. what is most likely true about the yellow solid?
Answers: 3

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1

Chemistry, 23.06.2019 10:30
Me soon im confused much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 1
You know the right answer?
An ideal solution is formed from a mixture of the nonvolatile solute urea, co(nh2)2, and methanol, c...
Questions

History, 19.11.2020 23:40



Mathematics, 19.11.2020 23:40

Social Studies, 19.11.2020 23:40



Health, 19.11.2020 23:40

Mathematics, 19.11.2020 23:40

Spanish, 19.11.2020 23:40

Health, 19.11.2020 23:40

Computers and Technology, 19.11.2020 23:40

Mathematics, 19.11.2020 23:40

History, 19.11.2020 23:40

Mathematics, 19.11.2020 23:40

Mathematics, 19.11.2020 23:40

Biology, 19.11.2020 23:40

English, 19.11.2020 23:40

Business, 19.11.2020 23:40