Chemistry, 10.07.2019 08:30 tybreyonnaHco7855
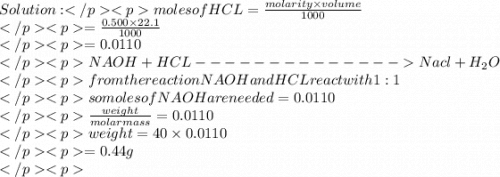
Asample contains both naoh and nacl. 0.500 g of this sample was dissolved in water to make a 20.0 ml solution and then this solution was titrated by 0.500 mol/l hcl solution. if 22.1 ml of hcl was used to reach the end point, what is the mass % of naoh in the sample?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
Asample contains both naoh and nacl. 0.500 g of this sample was dissolved in water to make a 20.0 ml...
Questions

Mathematics, 19.01.2021 23:20

Mathematics, 19.01.2021 23:20

Physics, 19.01.2021 23:30

Chemistry, 19.01.2021 23:30


Mathematics, 19.01.2021 23:30




Advanced Placement (AP), 19.01.2021 23:30


Biology, 19.01.2021 23:30

Mathematics, 19.01.2021 23:30

Mathematics, 19.01.2021 23:30

French, 19.01.2021 23:30


Mathematics, 19.01.2021 23:30