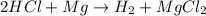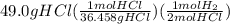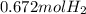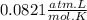When magnesium reacts with hydrochloric acid, hydrogen gas is formed: 2hcl + mg → h2 + mgcl2. what is the volume of hydrogen produced at 25°c and 101.3 kilopascals when 49.0 grams of hcl reacts with excess magnesium? use the periodic table and ideal gas resource. a. 1.38 l b. 2.76 l c. 16.4 l d. 32.9 l e. 33.1 l

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1


You know the right answer?
When magnesium reacts with hydrochloric acid, hydrogen gas is formed: 2hcl + mg → h2 + mgcl2. what...
Questions

Mathematics, 18.03.2021 01:00

English, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00



Mathematics, 18.03.2021 01:00





Advanced Placement (AP), 18.03.2021 01:00


Mathematics, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00

Biology, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00

Social Studies, 18.03.2021 01:00



Chemistry, 18.03.2021 01:00