Chemistry, 10.07.2019 06:00 Meap12345678910
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 3.75 ∘c. what is the molal concentration of glucose in this solution? assume that the freezing point of pure water is 0.00 ∘c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Asolution of water (kf=1.86 ∘c/m) and glucose freezes at − 3.75 ∘c. what is the molal concentration...
Questions


Social Studies, 24.06.2019 05:30




Social Studies, 24.06.2019 05:30








Physics, 24.06.2019 05:30

Mathematics, 24.06.2019 05:30

Mathematics, 24.06.2019 05:30

Mathematics, 24.06.2019 05:30

English, 24.06.2019 05:30


Biology, 24.06.2019 05:30

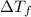 can be calculated as follows:
can be calculated as follows: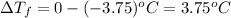
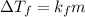
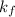 is freezing point depression constant.
is freezing point depression constant.