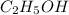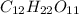Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2


Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1

You know the right answer?
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrol...
Questions

History, 02.10.2019 02:30

English, 02.10.2019 02:30

Mathematics, 02.10.2019 02:30

Mathematics, 02.10.2019 02:30

Mathematics, 02.10.2019 02:30


Mathematics, 02.10.2019 02:30









Mathematics, 02.10.2019 02:30

Social Studies, 02.10.2019 02:30



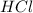
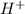 and
and 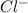 ) and thus, it is a strong electrolyte.
) and thus, it is a strong electrolyte.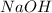
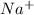 and
and 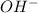 ) and thus, it is a strong electrolyte.
) and thus, it is a strong electrolyte.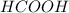
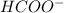 ) and thus, it is a weak electrolyte.
) and thus, it is a weak electrolyte.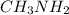
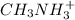 and
and 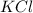
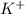 and
and