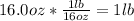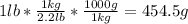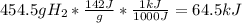Chemistry, 10.07.2019 06:00 coryintheswamp
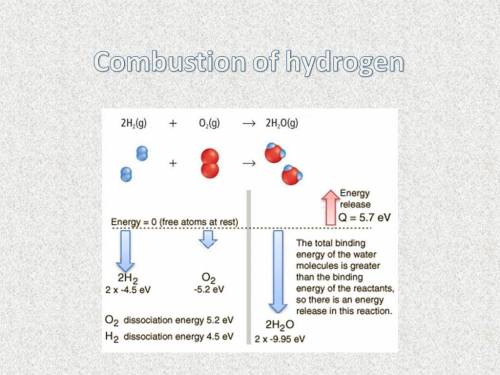
Combustion of hydrogen releases 142 j/g of hydrogen reacted. how many kj of energy are released by the combustion of 16.0 oz of hydrogen? (1 lb = 16 oz; 1 kg = 2.2 lb)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1
You know the right answer?
Combustion of hydrogen releases 142 j/g of hydrogen reacted. how many kj of energy are released by t...
Questions

English, 17.05.2021 02:10


English, 17.05.2021 02:10


History, 17.05.2021 02:10

Mathematics, 17.05.2021 02:10

Mathematics, 17.05.2021 02:20



Mathematics, 17.05.2021 02:20

History, 17.05.2021 02:20

Mathematics, 17.05.2021 02:20

English, 17.05.2021 02:20

Social Studies, 17.05.2021 02:20


Mathematics, 17.05.2021 02:20

Mathematics, 17.05.2021 02:20

Physics, 17.05.2021 02:20


History, 17.05.2021 02:20