

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 07:30
Assignment directions: pick one of the following chemists and perform a bit of research on him/her. answer the following questions. alice hamilton rosalind franklin marie curie gertrude b. elion ada yonath henry cavendish robert boyle antoine lavoisier mario j. molina svante arrhenius
Answers: 1

Chemistry, 23.06.2019 13:30
Use the periodic table to classify each of the elements below. cadmium (cd): vanadium (v): xenon (xe): iodine (i): potassium (k): strontium (sr):
Answers: 3

Chemistry, 23.06.2019 15:30
Which answer below correctly identifies the type of change and the explanation when magnesium comes into contact with hydrochloric acid
Answers: 1
You know the right answer?
An unknown liquid is composed of 34.31% c, 5.28% h, and 60.41% i. the molecular weight is 210.06 amu...
Questions

History, 29.01.2020 16:45

Physics, 29.01.2020 16:45



Mathematics, 29.01.2020 16:45

Geography, 29.01.2020 16:45

Mathematics, 29.01.2020 16:45




Chemistry, 29.01.2020 16:45









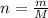
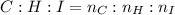
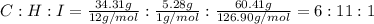
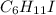 .
.

