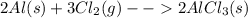Chemistry, 10.07.2019 05:30 Ciarrathereal
Solid aluminum metal and diatomic chlorine gas react spontaneously to form a solid product. give the balanced chemical equation (including phases) that describes this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Which one of the following gases is not an important component of soil?
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
Solid aluminum metal and diatomic chlorine gas react spontaneously to form a solid product. give the...
Questions


Geography, 29.01.2020 14:59

Mathematics, 29.01.2020 14:59

Business, 29.01.2020 14:59

Mathematics, 29.01.2020 14:59


Mathematics, 29.01.2020 14:59


Social Studies, 29.01.2020 14:59

Biology, 29.01.2020 14:59

Chemistry, 29.01.2020 14:59


Mathematics, 29.01.2020 14:59

Mathematics, 29.01.2020 14:59

Mathematics, 29.01.2020 14:59




English, 29.01.2020 14:59

Mathematics, 29.01.2020 14:59

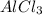 .
.