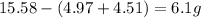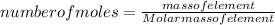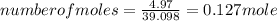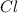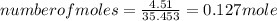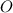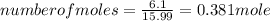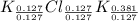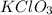Chemistry, 10.07.2019 05:30 geraldmorgan5580
A15.58 g sample of a compound contains 4.97 g potassium (k), 4.51 g chlorine (cl), and oxygen (o). calculate the empirical formula.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2


Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
A15.58 g sample of a compound contains 4.97 g potassium (k), 4.51 g chlorine (cl), and oxygen (o). c...
Questions

Chemistry, 14.10.2019 16:50



History, 14.10.2019 16:50





English, 14.10.2019 16:50



Mathematics, 14.10.2019 16:50

Social Studies, 14.10.2019 16:50

Biology, 14.10.2019 16:50


Chemistry, 14.10.2019 16:50

Mathematics, 14.10.2019 16:50



Computers and Technology, 14.10.2019 16:50