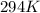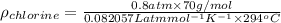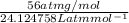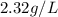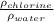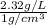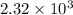Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Determine the specific gravity of chlorine (cl 2) gas at 21 degrees celsius [°c] and a pressure of 0...
Questions


History, 19.07.2019 20:30

Health, 19.07.2019 20:30

Biology, 19.07.2019 20:30


Business, 19.07.2019 20:30




Social Studies, 19.07.2019 20:30




Chemistry, 19.07.2019 20:30






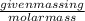
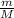 RT
RT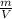 RT
RT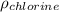 RT
RT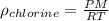
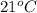 + 273 =
+ 273 =