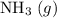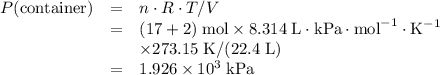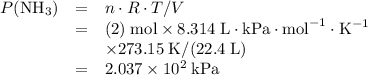Chemistry, 10.07.2019 05:30 sportsseolive4471
Avessel of volume 22.4 dm3 contains 20 mol h2 and 1 mol n2 ad 273.15 k initially. all of the nitrogen reacted with sufficient hydrogen to form nh3. calculate the total pressure and the partial pressure of each component in the final mixture at 273.15 k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
Avessel of volume 22.4 dm3 contains 20 mol h2 and 1 mol n2 ad 273.15 k initially. all of the nitroge...
Questions





Geography, 29.07.2019 08:30

History, 29.07.2019 08:30



History, 29.07.2019 08:30

Mathematics, 29.07.2019 08:30

Mathematics, 29.07.2019 08:30









History, 29.07.2019 08:30

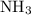 at a
at a 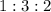 ratio:
ratio: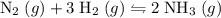
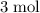 of hydrogen gas would have been consumed while
of hydrogen gas would have been consumed while 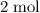 of ammonia would have been produced. The final mixture would therefore contain
of ammonia would have been produced. The final mixture would therefore contain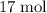 of
of 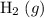 and
and