
Chemistry, 10.07.2019 05:30 anitadefrances
Use enthalpies of formation given in appendix c to calculate δh for the reaction br2(g)→2br(g), and use this value to estimate the bond enthalpy d(br−br).

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

You know the right answer?
Use enthalpies of formation given in appendix c to calculate δh for the reaction br2(g)→2br(g), and...
Questions



Social Studies, 23.08.2019 07:00



English, 23.08.2019 07:00


Social Studies, 23.08.2019 07:00



Biology, 23.08.2019 07:00


Biology, 23.08.2019 07:00




Physics, 23.08.2019 07:00

Mathematics, 23.08.2019 07:00

English, 23.08.2019 07:00

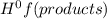 - ∑n(reactants)Δ
- ∑n(reactants)Δ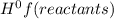
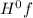 = enthalpy of formation
= enthalpy of formation

