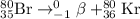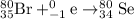Chemistry, 10.07.2019 05:30 22savage2017
He 80br (atomic number 35) nuclide decays either by β− decay or by electron capture. (masses of atoms: 80br =79.918528 amu; 80kr =79.916380 amu; 80se =79.916520 amu. neglect the mass of electrons involved because these are atomic, not nuclear, masses.) (a) write the balanced nuclear equations for each process below. use the isotope tool in the palette to enter both the mass numbers and the atomic numbers for each nuclide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 12:30
Nebulae are enormous clouds in outer space. they are made mostly of hydrogen gas, helium gas, and dust. some nebulae glow brightly, while others do not. the stars that people see are huge, bright balls of glowing gas. they are made mostly of hydrogen and helium. which statement correctly describes other ways in which nebulae and stars are different? a. stars can form inside a nebula but a nebula can never be produced by any star. b. a star always has a higher density than a nebula. c. stars can never form inside a nebula but a nebula can be produced by any star. d. a nebula always has a higher density than a star.
Answers: 3

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1
You know the right answer?
He 80br (atomic number 35) nuclide decays either by β− decay or by electron capture. (masses of atom...
Questions









Mathematics, 07.08.2019 04:10

Mathematics, 07.08.2019 04:10






English, 07.08.2019 04:10




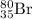 by
by 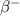 decay is as follows:
decay is as follows: