
Chemistry, 10.07.2019 04:00 briannasj6164
When copper crystallizes, it forms face-centered cubic cells. the unit cell edge length is 361.5 pm. calculate the density of copper in g/cm3?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Based on the law of conservation of energy, which statement is false? answer- energy is lost when machines dont work right
Answers: 1

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1
You know the right answer?
When copper crystallizes, it forms face-centered cubic cells. the unit cell edge length is 361.5 pm....
Questions



Computers and Technology, 29.01.2020 04:48







English, 29.01.2020 04:48



Mathematics, 29.01.2020 04:48

Mathematics, 29.01.2020 04:48




Mathematics, 29.01.2020 04:48


Mathematics, 29.01.2020 04:48

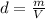
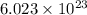 .
.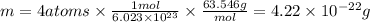
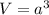
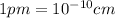
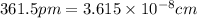
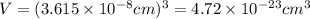
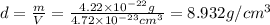
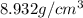 .
.

